 News & Resources
News & Resources
China announced it has granted conditional market approval for its first COVID-19 vaccine during a Thursday news conference.What is the development and production process of the new crown inactivated vaccine? How to strictly control vaccine quality through refined production, storage, and transportation processes?

The vaccine also has an ID card?
Each dose of vaccine is equipped with a supervision code that traces the flow of the whole process. The supervision code is equivalent to the "electronic ID card" of the vaccine. Through the supervision code, the process of vaccine production, storage, and transportation can be inquired, and the person receiving the injection can be traced.
Light inspection
In the fully automatic prefilling packaging line, the filled vaccine must first undergo light inspection. This step is to use a high-speed camera to replace the human eye to observe whether there are products containing particles, foreign matter, glass slag and defective filling volume, and remove them.
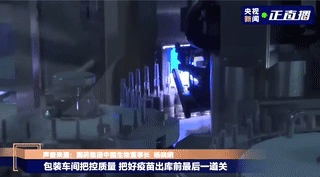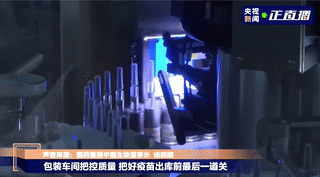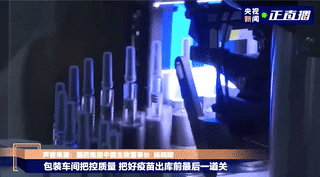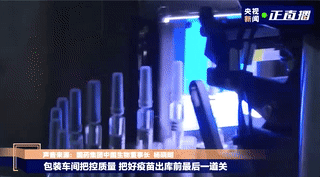
Labeling
Vaccines that pass the light inspection will be labeled and the syringe plunger will be installed. The label is marked with information such as the product name, production date, and serial number. The medical staff can directly inject the vaccine when they get it, without drawing the vaccine into the syringe.

Boxing
The vaccine enters the boxing process. Each vaccine is equipped with an instruction manual, and online weighing will be carried out after the packaging is completed. Once the weight is found to be incorrect, such as lack of instructions or putters, they will be eliminated, adding another layer of protection to vaccine safety.

Coding
The regulatory code is equivalent to the "electronic ID card" of vaccines, which is divided into three levels: each vaccine is individually coded, every 10 vaccines is composed of a medium package, and the large package contains multiple medium packages and then coded. Through the supervision code, you can check whether the vaccine production, warehousing, storage, delivery, transportation and other processes meet the standards, and track the person receiving the injection.

How to transport and store?
The interim results of its phase-3 clinical trials show 79.34 percent efficacy against COVID-19, meeting the standards of the World Health Organization and the NMPA, according to a press conference by the State Council joint prevention and control mechanism against COVID-19.This vaccine will be provided free of charge to all Chinese people.
